Objectives:
1. To synthesize azo dyes
2. To understand the formation of azo dyes
3. To understand how to prepare a dye
Materials:
Sodium nitrite, concentrated HCl, sodium hydroxide solution, sodium chloride, 4-nitroaniline, salicylic acid, white cotton fabric
Apparatus:
Test tube, ice bath, vacuum filtration apparatus, glass funnel, and hotplate
Procedures:
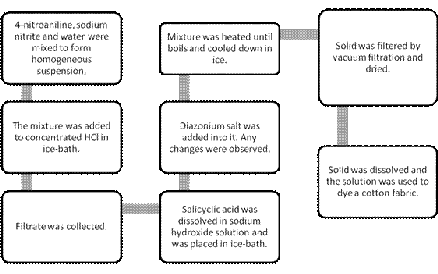
Results and calculations:
Table 1: Observation of dye colour changes
| Observations | |
| During synthesis | When the darkish green slurry is added into hydrochloric acid, the mixture turns to darkish red. |
| During dyeing | The cotton fabric is dyed with darkish red but fades to pale brown. |
Table 2: Mass of solid dye
| Mass of filter paper | 0.3278g |
| Mass of (filter paper + solid dye) | 1.9518g |
| Mass of solid dye | 1.6240g |
Calculating percentage yield
Mole number of 4-nitroaniline = 0.7066g/ 138.12g mol-1
= 0.0051 mol
Mole number of sodium nitrite = 0.3800g/ 69g mol-1
= 0.0055mol
Thus, 4-nitroaniline is the limiting agent in the first reaction.
Mole number of salicylic acid = 0.68g/ 138.12g mol-1
= 0.0049 mol
In the second reaction, salicylic acid is the limiting agent.
Theoretical mole number of diazo compound = 0.0049mol
Actual mole number of diazo compound = 1.6240g/ 287.12g mol-1
= 0.0057mol
Percentage yield = 0.0057 mol/ 0.0049mol x 100%
= 116.33%
Discussion:
The purpose in this experiment is to synthesize azo dye and dye it on a cotton fabric. The colour of azo dye formed in this experiment was darkish red. However, it faded to pale brown after a few minutes. The amount of diazo compound obtained is 1.6240g with the percentage yield of 116.33%. The percentage yield is over 100% might be due to the presence of unreacted reagents.
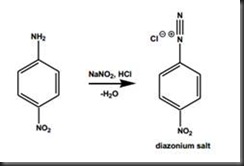
In the synthesis of diazonium salt, sodium nitrite and 4-nitroaniline were mixed in water and then the slurry was added into concentrated hydrochloric acid. The darkish green solution was formed via the reaction as shown in diagram 1 below:
Diagram 1 Formation of diazonium salt
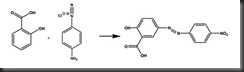
The azo dye was formed by further react with certain aromatic compound such as salicylic acid in this experiment via the process called coupling. The darkish red azo compound was formed as the following reaction of salicylic acid and diazonium salt.
The synthesized diazonium salt and azo compound showed their own colour because each compound contains aromatic ring and the aromatic allows the delocalisation of electron to occur. This delocalized electron system is capable to absorb different wavelength of light and hence each compound showed different colours.
Precaution steps:
1. Handle 4-nitroaniline carefully because it is highly toxic compound.
2. Avoid skin contact with sodium nitrite. It is toxic oxidiser.
3. Wear gloves when handling the dyes.