Objective:
1. To learn the separation technique by using thin layer chromatography plate in separating a mixture of compounds into individual pure compound
2. To separate the components of maker ink
3. To separate the components of food dye
4. To separate the pigments that present in the spinach via thin later chromatography
Introduction:
The name called chromatography stems from an experiment in which a mixture of coloured (chroma) compounds from a plant extract was separated on paper so that the individual colours were written (graphy) for all to see. Chromatography is one of the most important techniques used in chemistry. It is used to purify desired products from contaminating reaction by products and to isolate biologically active compounds like new drugs from natural sources. Chromatography is also used in chemical analysis. Some examples are the detection of pesticide residues in food, soil and water, prohibited drugs in the blood of athletes and drivers and inorganic ions like nitrate and nitrite whose presence in natural waters can lead to toxic algal blooms.
Chromatography is a common laboratory technique to separate and analyze two or more analytes in the mixture by distribution of two phases: a stationary phase and a mobile phase. The stationary phase is a phase which allows the mobile phase to travel along. These two phases can be solid-liquid, liquid-liquid or gas liquid. This method works on the principle that different compounds with different solubilities and adsorptions to the two phases which they are to be partitioned. The stationary phase bonds to compounds by weak reversible intermolecular interactions (eg. electrostatic, Van der Waals interactions or hydrogen bonding) but most importantly, the strengths of these bonds depend on the structure and polarity of the individual compounds. This is means that when there is a competition between compounds and the bonding sites on the stationary phase some of the compounds will be held more strongly than others.
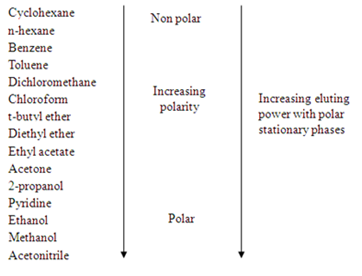
The compounds in the mobile phase will have different interaction with the polar stationary phase. The factors are mainly depends on the polarity of adsorbent (silica gel in this experiment), solvent polarities, and functional groups of the compounds. The polar adsorbent will more strongly attract the polar molecules of compounds and it will have lower affinity to the non-polar compounds. Hence, the movement of compounds with different polarities could be different. In addition, the polarity of solvent is very important to the compound separations, a solvent system may increase in its polarity by changing the composition of the solvent mixture. The more polar the solvent, the faster the compounds can be drawn up, which means the further the compounds move. The comparison of the polarities of solvent are listed down in the diagram 1.
Diagram 1
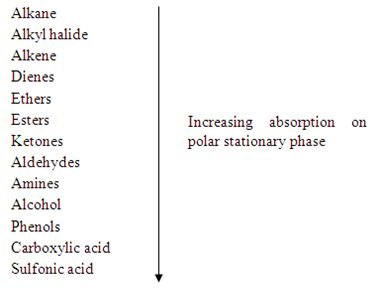
The second factor that affects the interaction between stationary phase and compounds is functional group of each compound. The highly polar groups in compound will cause the stronger adsorption and eluted less readily to the stationary phase compared to less polar compounds. Hence, the highly polar compounds will tend to interact strongly with the polar adsorbents and absorb onto the fine particles of the absorbent, hence it travel slowly. The adsorption strengths of each compound having the following types of functional groups in the order of increasing group polarities.
However, the variation may take place which depends on the overall structure of each compound.
In a typical procedure, a mixture of compounds is applied to one end of a plate or column of stationary phase. Then a mobile phase which can carry the compounds in the mixture is allowed to run over or through stationary phase, starting from the end at which the compounds were applied and going to other. As the mobile phase runs, the compounds with strongest bonds to the stationary phase continually displace the others which are then carried further up the chromatography. Consequently, the most weakly bonded molecules end up furthest along the plate and as times goes on, the individual compounds are all separated from each other.
In this experiment, the mixture of the compounds are applied to the bottom of plate or sheet which holding a thin layer of the stationary phase. The mobile phase is allowed to move along the plate by capillary action to develop the chromatography. The compounds are visualized and marked on the plate. In order to visualize the compounds, ordinary visible light is used for coloured compounds, UV light is used for compounds that contain double bonds, and the certain reagent (iodine) is used to react with the separated compounds to produce coloured spots.
Finally, the position of each spot on the plate is measured and reported as the retention factor (or retardation factor), Rf value. Retention factor is the ratio of distance traveled of the compound to the distance of the solvent traveled. Retention factor, Rf is the distance of compound traveled divided by the total distance of solvent travelled in TLC plate. The Rf value are important because they are a consistent property of a compound so long as the stationary and mobile phases are identical. The Rf value can assist in identifying a compound. If two spots travel the same distance on the TLC plate or they have the same retention factor, then both compounds might be concluded as the identical compound.
Materials:
Commercial felt tipped writing pen, spinach, sand, chloroform (CH2Cl2), methanol (CH3OH), petroleum spirit, ethanol (CH3CH2OH), 2M ammonia solution, 1-butanol (C8H10OH), acetone
Apparatus:
Capillary tubes, mortar and pestle, Merck TLC plate, Whatman 12.5cm filter paper, Pasteur pipette, ultra-violet light (long wave length and short wave length)
Procedure:
Part I: Separation of inks from a commercial felt tipped writing pen
Prepare the developing tank
1. 6ml of 1-butanol, 2ml of ethanol and 2ml of 2M ammonia solution were prepared into two 150cm3 beakers.
2. A filter paper was inserted into each beaker and the solution was swirled to wet the paper.
3. Each beaker was covered by a watch glass.
Spot the plate
1. 8.5cm x 3cm of chromatography paper (Whatman SG 81) and 10cm x 3.5cm TLC plate were obtained which can fit inside the developing tank without touching the watch glass.
2. For filter paper and TLC plate, two dots with 1cm apart and 1.2cm from the bottom edge were made by pencil. The dots were labeled as “1” and “2”. The sample (marker ink 500) was spotted on the spot marked “1” once and three times on the spot marked “2”.
3. Steps 1 and 2 were repeated by using marker ink 500A and food dye (red).
Develop the chromatogram
1. The chromatography paper and TLC plate were placed into the different developing tank and make sure that the spots are above the solvent level.
2. The beakers were covered with glass watch.
3. The solvent was allowed to travel upwards.
4. The filter paper and TLC plate were removed and the maximum height of the eluent reached was marked with pencil.
Record the result
1. An accurate drawing was made to show the identity of the sample, the type of stationary phase, the mobile phase, the colour of each discernible spot, the method of visualization and the Rf value for each compound.
Part II: Separation of spinach leaf pigments by TLC
Prepare the developing tank
1. 10ml mobile phase was prepared from the mixture of 9.5ml CH2Cl2 and 0.5ml CH3OH.
2. The mixture was poured into a 150cm3 developing tank.
3. A filter paper was inserted and the mobile phase was swirled to wet the filter paper.
4. The beaker was covered by a watch glass.
Extraction procedure
1. 10g of spinach was grinded by using a mortar and pestle with a portion of sand as extra abrading agent.
2. 20ml of acetone was added and the sample was grinding until the extract is green and the spinach is completely pulped.
3. 10ml petroleum spirit was added and it was mixed quickly with the sample by using pestle.
4. A Pasteur pipette was used to transfer 5ml of the liquid part of the mixture into a 10cm3 glass vial.
5. The vial was capped immediately.
Spot the plate
1. A 1.5cm x 6.6cm TLC plate was used and a dot was made by using a pencil from 1.2cm from the bottom.
2. The green layer of spinach was spotted on the plate several times.
Develop the chromatogram
1. The TLC plate was placed into the developing tank.
2. The solvent front was marked.
Record the result
1. The plate was observed under UV light and the observation was recorded.
2. Accurate drawing of TLC obtained was drawn to show the identity of the sample, the type of stationary phase, the mobile phase, the colour of each discernible spot, the method of visualization and the Rf value for each compound.
Results and calculations:
Based on the formula below to calcuate the retention factor of each component in the sample.
Rf is refers to the retention factor.
Observation:
Observation:
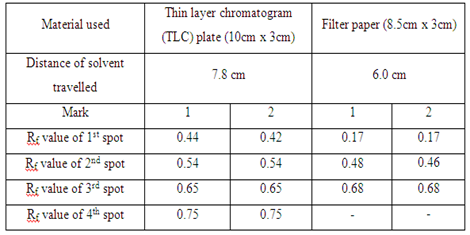
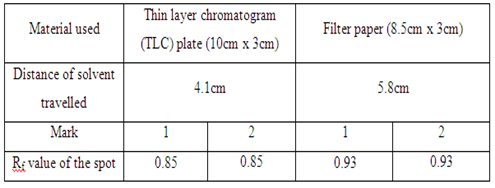
| Material used | Thin layer chromatogram (TLC) plate (10cm x 3cm) | Filter paper (8.5cm x 3cm) |
| Distance of solvent travelled | - | - |
Observation:
Observation:
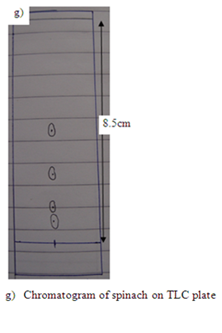
| Material used | Thin layer chromatogram (TLC) plate (10cm x 3cm) |
| Distance of solvent travelled | 8.5cm |
| Rf value of 1st spot | 0.09 |
| Rf value of 2nd spot | 0.15 |
| Rf value of 3rd spot | 0.31 |
| Rf value of 4th spot | 0.49 |
| Number of spot | Rf value | Colour observed |
| 1st spot | 0.09 | Yellow |
| 2nd spot | 0.15 | Yellow |
| 3rd spot | 0.31 | Yellow |
| 4th spot | 0.49 | Green |
Discussion:
In the thin layer chromatography, the eluent (solvent) is prepared by using a mixture of 1-butanol, ethanol and ammonia solution in the ratio of 6:2:2. The polarity of the particular solvent cannot be too low because the polar compounds will not be able to carry by the eluent and will not be separated, so that the separation might not be observable. If the solvent of too high polarity is used, the polar compound will travel so fast that the separation between non-polar compound and polar compound to become so small and poor separation will be observed. The solvent mixture is believed that it has the optimized solubility for the organic compounds to dissolve in the solvent. In another word, the compounds can be easily to be carried by the solvent in the TLC plate and filter paper. Before the plate is placed into the solvent, a filter paper was dipped inside the solvent in a beaker which is covered by an evaporating flask. This is to create a system that full with the vapours of organic solvent and hence the solvent is allowed to travel up along the plate faster. After the plate was introduced into the solvent, the solvent start to migrate itself and the compounds move along with solvent until the solvent front has been reached.
There are three components in the chromatography study in which includes the TLC plate with adsorbent or filter paper, the development solvent and the organic compounds that to be analyzed. The adsorbent, silica gel consists of a three dimensional network of thousands of alternating silicon and oxygen bonds. It is a very polar and is capable of hydrogen bonding due to its partial positive charge in silicon and partial negative in oxygen. The silica gel with compete with the development solvent for the organic compounds as the solvent is traveling up through the TLC plate. The silica gel tends to bind the compounds (on stationary phase) while the development solvent tried to dissolve the compounds (on mobile phase) in order to carry the compounds along the plate as the solvent travels up. All the compounds are possible to be adsorbed into the stationary phase however the time of adsorption of compounds in the particular phase is depends on the polarity of each compound. The more polar the compound is, the longer the time taken that the compound adsorbed into the stationary phase so it eluting speed is slower (more time on stationary phase). Less polar compounds are weakly adsorbed, so the time taken for less polar compounds to be adsorbed on stationary phase is shorter. As a result, the less polar compounds can travel further along the plate compared to the more polar compounds. In the paper chromatography, the organic compounds also tend to move upward and the mobile phase carries the organic compounds to run over or through the stationary phase. However, the paper chromatography might not be suitable for some compounds since the compound separation is not good enough in certain case.
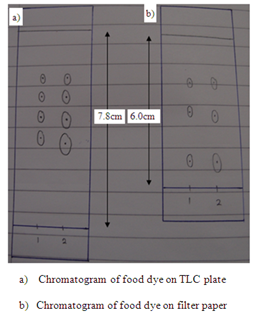
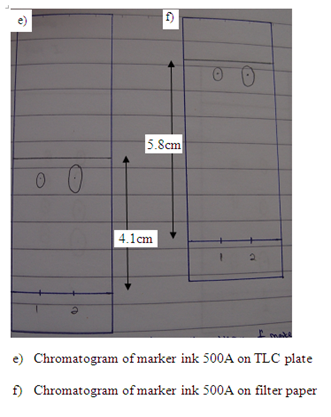
In the first part, the food dye (red colour), marker ink 500, and marker ink 500A were used in the study of the chromatography. In the chromatogram a, the food dye were applied one time and three times separately in two different spots (marked as 1 and 2 respectively). The result obtained showed that the distances of each component in food dye moved are almost identical in the TLC plate. This proved that the number of sample spotted on the plate did not affect the distance of each component in food dye travelled in TLC plate. However, the separation of compound in filter paper was not good. This is because there are only three spots appeared on the filter paper (which is shown in chromatogram b) while TLC plate has four single component. In chromatogram c and chromatogram d, there is no spot being observed in both chromatograms. This might be due to the low polarity of sample ink marker 500 in which the low polar organic compounds cannot move in either TLC plate or filter paper. By comparing the chromatogram e and chromatogram f, both chromatograms have one similarity in which they showed that only one component present in the ink marker 500A. Two spots (one spot is being spotted once and another one spot is being spotted three times) in both chromatograms shows the similar Rf value and hence two compounds can be concluded to they migrated the same distance in each chromatogram.
In the second part of the experiment, the spinach was being applied on a TLC plate. The TLC plate showed there are total four components in spinach. The Rf value of the first spot, second spot, third spot, and forth spot are 0.09, 0.15, 0.31, and 0.49 respectively. The colours of each spot in the TLC plate have been recorded, which the compounds with Rf value of 0.09, 0.15 and 0.31 showed yellow colour while the forth spot showed green colour. The yellow spot are predicted as the xanthophylls while the green spot is predicted as chlorophyll. Xanthophylls and chlorophylls are the pigments that usually can be found in the plant
Safety concerns:
1. Always wear goggles and gloves.
2. Handle and dispose the mobile phase into the waste bottle.
3. Do not shake or disturb the mobile phase after TLC plate has been introduced into the beaker.