Title: Determination of the critical micelle concentration (CMC) of an amphiphile by conductivity method
Objective:
1. To learn the process involved in CMC determination of a surfactant
2. To determine the critical micelle concentration of the amphiphile sodium dodecyl sulphate (SDS)
Introduction:
Surfactants are amphiphilic molecules that possess both hydrophobic and hydrophilic properties. A typical surfactant molecule consists of a long hydrocarbon ‘tail’ that dissolves in hydrocarbon and other non-polar solvents, and a hydrophilic ‘headgroup’ that dissolves in polar solvents (typically water). One example of a dualcharacter molecule having a head-group and a non-polar tail is sodium dodecyl sulphate (SDS), Na+ -OSO3Cl2H25. When a sufficient amount of SDS is dissolved in water, several bulk solution properties are significantly changed, particularly the surface tension (which decreases) and the ability of the solution to solubilise hydrocarbons, (which increases). These changes do not occur until a minimum bulk SDS concentration is reached. This concentration is called the critical micelle concentration (CMC).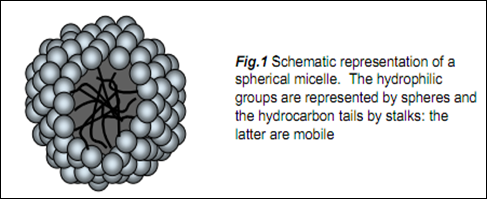 Several experiments, including light scattering and NMR, show that below the CMC, the surfactant exists mainly as solvated monomeric species, whereas above the CMC these monomers undergo self-assembly to form roughly spherical structures (having an overall diameter of ~5 nm) known as micelles (see Fig 1). Micelles are the simplest of all self-assembly structures.
Several experiments, including light scattering and NMR, show that below the CMC, the surfactant exists mainly as solvated monomeric species, whereas above the CMC these monomers undergo self-assembly to form roughly spherical structures (having an overall diameter of ~5 nm) known as micelles (see Fig 1). Micelles are the simplest of all self-assembly structures.
Technically, a micellar solution is a colloidal dispersion of organised surfactant molecules. Non-ionic surfactant molecules can cluster together in micelles of 1000 molecules or more, but ionic species tend to form micelles of between 10 and about 100 molecules because of electrostatic repulsions between head-groups. One of the key aspects of micelle structure is that the interior of the micelle consists of an associated arrangement of hydrocarbon chains (an ‘oil droplet’). The exterior coat is constructed of the polar, ionic moieties (the OSO3- groups in the case of SDS). This ionic surface (which also contains associated water of hydration) is called the Stern layer. Surrounding this ionic mantle is a region that contains both counterions and oriented water molecules – the Gouy-Chapman layer. Together the Stern and Gouy-Chapman layers are known as the electrical double layer. But it is the oil-like interior of the micelle that gives it its many diverse and interesting properties. The hydrocarbon core (~3 nm in diameter) has the capacity to accommodate guest molecules. The most common application of micelles is as detergents but they can also act as micro-reaction vessels for organic syntheses and drug delivery agents
In this experiment you will determine some fundamental properties of the SDS micelle: the CMC and the free energy, enthalpy and entropy of micellisation. You will measure the CMC by measuring the conductivity of the system as a function of SDS concentration. The thermodynamic properties are obtained by determining the CMC at a variety of temperatures. You will need to pool your data – each member of the team will determine the CMC at a different temperature.
Conductometric Determination of the CMC
Below the CMC, the addition of surfactant to an aqueous solution causes an increase in the number of charge carriers ( (aq) Na+ and (aq) -OSO3Cl2H25 ) and consequently, an increase in the conductivity. Above the CMC, further addition of surfactant increases the micelle concentration while the monomer concentration remains approximately constant (at the CMC level). Since a micelle is much larger than a SDS monomer it diffuses more slowly through solution and so is a less efficient charge carrier. A plot of conductivity against surfactant concentration is, thus expected to show a break at the CMC (Figure 1).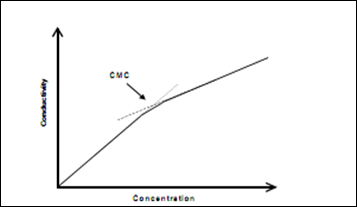 Figure 1
Figure 1
Apparatus: beaker, pipette, conductivity meter, glass rod
Materials: SDS, deionised water
Procedure:
1. 50ml of an approximately 0.04M aqueous stock solution SDS was prepared.
2. 25ml of deionised water was pipetted into a 200ml beaker.
3. 0.5ml of SDS stock solution was pipetted into water and stir.
4. The conductivity was recorded.
5. Repeat steps 3 and 4 until all the SDS have been added into the beaker.
6. A conductivity as a function of the SDS concentration was plotted and CMC was estimated.
7. The standard change in Gibbs free energy was calculated.
Results and calculation:
| Volume of Stock Solution of SDS added, V1 (ml) | Concentration of SDS in solution, M2 (M) | Conductivity, (mS) |
| 0.0 | 0.0000 | 9.17 |
| 0.5 | 0.0007 | 27.2 |
| 1.0 | 0.0015 | 56.1 |
| 1.5 | 0.0023 | 67.3 |
| 2.0 | 0.0030 | 102.8 |
| 2.5 | 0.0036 | 108.4 |
| 3.0 | 0.0043 | 96.5 |
| 3.5 | 0.0049 | 137.8 |
| 4.0 | 0.0055 | 99.6 |
| 4.5 | 0.0061 | 160.4 |
| 5.0 | 0.0067 | 207.0 |
| 5.5 | 0.0072 | 204.0 |
| 6.0 | 0.0077 | 238.0 |
| 6.5 | 0.0083 | 247.0 |
| 7.0 | 0.0088 | 257.0 |
| 7.5 | 0.0092 | 245.0 |
| 8.0 | 0.0097 | 253.0 |
| 8.5 | 0.0101 | 272.0 |
| 9.0 | 0.0105 | 286.0 |
| 9.5 | 0.0011 | 301.0 |
| 10.0 | 0.0114 | 307.0 |
| 10.5 | 0.0118 | 313.0 |
| 11.0 | 0.0122 | 322.0 |
| 11.5 | 0.0126 | 331.0 |
| 12.0 | 0.0130 | 337.0 |
| 12.5 | 0.0133 | 344.0 |
| 13.0 | 0.0137 | 348.0 |
| 13.5 | 0.0140 | 354.0 |
| 14.0 | 0.0144 | 357.0 |
| 14.5 | 0.0147 | 365.0 |
| 15.0 | 0.0150 | 371.0 |
| 15.5 | 0.0153 | 375.0 |
| 16.0 | 0.0156 | 380.0 |
| 16.5 | 0.0159 | 384.0 |
| 17.0 | 0.0162 | 389.0 |
| 17.5 | 0.0165 | 393.0 |
| 18.5 | 0.0170 | 408.0 |
| 19.5 | 0.0175 | 418.0 |
| 20.5 | 0.0180 | 422.0 |
| 21.5 | 0.0185 | 425.0 |
| 22.5 | 0.0190 | 434.0 |
| 23.5 | 0.0194 | |
| 24.5 | 0.0198 | 445.0 |
| 26 | 0.0204 | 454.0 |
| 27 | 0.0208 | 459.0 |
| 28 | 0.0211 | 464.0 |
| 29 | 0.0215 | 473.0 |
| 30 | 0.0218 | 475.0 |
| 31 | 0.0221 | 480.0 |
| 32 | 0.0225 | 483.0 |
| 33 | 0.0228 | 493.0 |
| 34 | 0.0231 | 714.0 |
| 35 | 0.0233 | 879.0 |
| 36 | 0.0236 | 907.0 |
| 37 | 0.0239 | 946.0 |
| 38 | 0.0241 | 964.0 |
| 39 | 0.0244 | 975.0 |
| 40 | 0.0246 | 988.0 |
| 41 | 0.0248 | 996.0 |
| 42 | 0.0251 | 1004.0 |
| 43 | 0.0253 | 1014.0 |
| 44 | 0.0255 | 1018.0 |
| 45 | 0.0257 | 1021.0 |
| 46 | 0.0259 | 1025.0 |
| 48 | 0.0263 | 1035.0 |
| 49 | 0.0265 | 1041.0 |
| 50 | 0.0267 | 1055.0 |
Graph 1 Conductivity against SDS concentration in the solution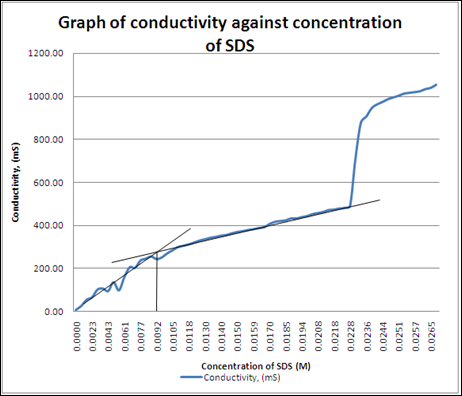
At the critical micelle concentration (CMC), the conductivity of the solution is approximately 100, hence the concentration of the SDS solution is approximately 0.003M. and provided the value of p/n = 0.3.
ΔG M, m° ≈ RT (2 – p/n) ln [CMC]
= 8.3145 J mol-1 K-1 x (25 + 273) K x (2 – 0.3) ln 0.092
= - 10.05 kJ mol-1
Discussion:
In this experiment, the critical micelle concentration (CMC) of sodium dodecyl sulfate was determined by using the method of conductivity. Sodium dodecyl sulfate (SDS), NaOSO3C12H25 is known as amphiphilic surfactant which possesses both hydrophobic and hydrophilic properties. SDS was ionized in the aqueous solution to form Na + and -OSO3C12H25 ions in the solution. Self-dissociation of SDS into micelle is strongly cooperative and occurs at the defined concentration called critical micelle concentration. Below CMC, the amphiphile dissolves as monomers. Once the concentration beyonds CMC, the monomers concentration remains unchanged while the micelle concentration increases. The CMC can be determined by the conductivity method of the SDS solution. Na + and -OSO3C12H25 ions are known as charge carriers which will increase the conductivity of the solution when ionization takes place.
At the beginning of the experiment, a small amount of SDS is added into the distilled water. In a SDS dilute solution, the concentration of SDS is below its CMC, hence it behaves as normal electrolyte and ionizes to give out Na + which soluble in the aqueous phase while -OSO3C12H25 ions solubilize its hydrophilic head in the water and hydrophobic tail extent out the water surface. The ions exist as solvated monomer instead of micelle due to low SDS concentration. The number of monomers was increased as the amount of the SDS solution was added into the solution. At the same time, the increase of conductivity that had been detected due to the increase of SDS ions carried more charges within the solution. Once the amount of SDS solution added into the aqueous solution is equals to the CMC, the first micelle start to form spontaneously in the solution.
The micelle formation occurs at the above of CMC which the monomers undergo self-assembly to form aggregate in the solution. This caused the solution converted from true solution to become a colloidal system. The micellar solution is known as a colloidal dispersion (association colloid) of organized surfactant molecules. The micelle formed in the solution is a spherical structure which the hydrophilic head groups were exposed to the solution while the hydrophobic tails were faced toward the interior of the micelle structure. The exterior of the micelle is built up from the ionic –OSO3 groups which form the Stern layer which associated by water molecules. The further layer that surrounding the Stern layer is composed of the positive counter ions and oriented water molecule called Gouy-Chapman layer. Both Stern layer and Gouy-Chapman layer are known as electric double layer. This double layer will maintain the stability of the colloidal system.
The higher concentration of SDS caused nucleation for the micelle to form increased and hence more micelle was formed in the solution. Above the CMC, the concentration of micelle definitely increases. However, the concentration of monomers almost remained unchanged in the solution. Monomers tend to form the micelle at the same time the added SDS solution ionized in the solution to replace the monomers that used to build micelle. But, the charge carriers could be increased slowly because the rate of micellisation is slower than the rate of monomers were used in the building of micelle and hence the conductivity of the solution increased at a slower rate in an ideal condition. This can be noticed in the graph 1 which shows the increasing rate of conductivity had became slower obviously. This is because the formation of micelle required the ionic monomers and some of the ions had been attracted towards the micelle surrounding to form the electric double layer. As a result, some monomers are no longer free in the solution but for those ions are not strongly attracted still can carry charge in the solution. Hence, the conductivity of the solution increased slower. However, at the final part in graph shows a sudden increase in the conductivity of the may be due to the formation of bubbles inside the solution. Above the CMC, when bubbles start forming, micelles will be broken down to form monomers to expand the bubbles. As more SDS monomers being formed back, the conductivity shoot up because SDS monomers is a more effective charge carrier than micelles.
Precaution Steps
1. The stirring is controlled not to be too fast during the experiment to avoid the formation of bubbles as bubbles can affect the conductivity.
2. SDS solution is added slowly to the water to prevent the formation of bubbles.